Quality Management System
GMP for Dietary Supplements
・To prevent human error during the manufacturing processes
・To prevent the contamination and degradation of products
・To aim for homogenization of every product
We achieve the three objectives mentioned above by creating appropriate frameworks implementing operational management from the receipt of raw materials, to the distribution of the final product. Our framework covers both “software” systems, such as quality analysis and quality control, and “hardware” systems, such as the utilization of appropriate buildings, facilities, culturing tanks, machines, and so on. This framework allows us to make sure that all products we manufacture are always of the highest possible quality.
Acquired in 2019.
Fermented foods are garnering lots of attention these days as a health food. Quickly realizing their power, our company has become a pioneer. With more than 90 patents and over 100 published papers and presentations at academic conferences within Japan, the fermented foods developed at Nodashokukin Kogyo Co., Ltd. contain many unknown powers that have yet to be elucidated. Our ability to propose new products and our wide research and development network. Total backup for the health food industry. Our aim is to create a variety of backup systems, from product development by researchers at our own laboratory and sales and support by our sales staff, to the pursuit of evidence by our network of medical and public institutions.
At Nodashokukin, we value the science and evidence behind our ingredients. The data we have also made it possible for us to patent the raw materials, and through scientific papers, we are striving to ensure the reliability and reliability of our products.

GMP for Dietary Supplements
・To prevent human error during the manufacturing processes
・To prevent the contamination and degradation of products
・To aim for homogenization of every product
We achieve the three objectives mentioned above by creating appropriate frameworks implementing operational management from the receipt of raw materials, to the distribution of the final product. Our framework covers both “software” systems, such as quality analysis and quality control, and “hardware” systems, such as the utilization of appropriate buildings, facilities, culturing tanks, machines, and so on. This framework allows us to make sure that all products we manufacture are always of the highest possible quality.
Acquired in 2019.
Fermented foods are garnering lots of attention these days as a health food. Quickly realizing their power, our company has become a pioneer. With more than 90 patents and over 100 published papers and presentations at academic conferences within Japan, the fermented foods developed at Nodashokukin Kogyo Co., Ltd. contain many unknown powers that have yet to be elucidated. Our ability to propose new products and our wide research and development network. Total backup for the health food industry. Our aim is to create a variety of backup systems, from product development by researchers at our own laboratory and sales and support by our sales staff, to the pursuit of evidence by our network of medical and public institutions.
At Nodashokukin, we value the science and evidence behind our ingredients. The data we have also made it possible for us to patent the raw materials, and through scientific papers, we are striving to ensure the reliability and reliability of our products.


Manufacturing Dvision
Manufacturing Dvision
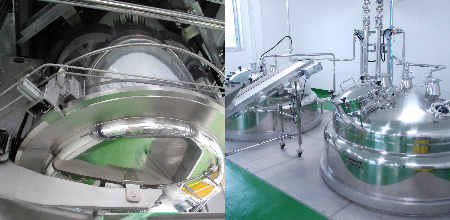
Production at Our Factory
Safe, reliable, and high quality. In order to ensure customer satisfaction, each individual staff member has a commitment to quality and manufactures all products in accordance with our policies.
Medical-grade Microbial Control

Traceability
Product records such as raw material used, facilities, and even down to which particular tank was used are all kept. All records are preserved under a strict record management system. In the case of any problems arising with a product, this allows us to smoothly identify the cause, or take steps to ensure the same issue does not occur again.
Pre-shipment Checks
All products are re-quality tested upon batch completion, and only products that pass leave our factory. Each test is specialized to the form and characteristics of each product and are carried out to the strictest standards.